number of electrons in one coulomb of charge|How many electrons are there in 1 coulomb? : Pilipinas Solution. Step 1: Given data. To figure out how many electrons make up one coulomb of charge, e = 1 .6 × 10 − 19 C. ∴ q = 1 C. Step 2: Concept used. An electron's charge is negative and is 1.6 × 10 - 19 coulomb. Electrons constituting one coulomb of charge, . #HerbalMed#Sampa-sampalukan©Kalusugan-phDisclaimer: Ang mga halamang gamot ay maaaring makatulong sa iba’t ibang mga karamdaman. Subalit marami dito ay wala .
PH0 · electrostatics
PH1 · How to Determine the Number of Electrons on an
PH2 · How many electrons are there in 1 coulomb?
PH3 · Electric charge
PH4 · Electric Charge
PH5 · Coulomb
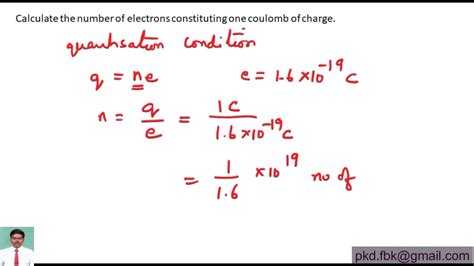
PH6 · Calculate the number of electrons constituting one coulomb of charge
PH7 · Calculate the number of electrons constituting one coulomb of
PH8 · Calculate the number of electrons constituting one
Past Announcements. Advisory for Transfer and Re-Admission to PUP Main Campus Posted: 08/07/2024; Schedule of Online Registration for Summer, Academic Year 2023-2024, and Online Encoding of Grades for 2023-2024 Second Semester Posted: 07/03/2024; Invitation to Submit Proposals or Quotations for Academic Gown Toga .
number of electrons in one coulomb of charge*******Solution. Step 1: Given data. To figure out how many electrons make up one coulomb of charge, e = 1 .6 × 10 − 19 C. ∴ q = 1 C. Step 2: Concept used. An electron's charge is negative and is 1.6 × 10 - 19 coulomb. Electrons constituting one coulomb of charge, .Physics. Question. Calculate the number of electrons constituting one coulomb of charge. Solution. Verified by Toppr. we know that the magnitude of charge on one electron .
The web page provides a verified solution to the question of how many electrons are there in 1 coulomb of charge. It also shows similar questions and answers related to the topic .Ene 17, 2024 — The number of electrons constituting one coulomb of charge can be calculated using the elementary charge, denoted as “e,” which is approximately equal to .The coulomb (symbol: C) is the unit of electric charge in the International System of Units (SI). It is equal to the electric charge delivered by a 1 ampere current in 1 second and is defined in terms of the elementary charge e, at about 6.241509×10 e.
number of electrons in one coulomb of charge How many electrons are there in 1 coulomb? Hul 23, 2018 — In a textbook I found it is stated that - " 1 coulomb is that quantity of electric charge which exerts a force of 9 ×10^9 newtons on an equal charge placed at a .Coulomb: Coulomb is the unit used to quantify charge. 1 Coulomb is equivalent to the charge carried by 1.6 × 10 − 19 protons. Let us test our understanding by going over two.A Coulomb equals the charge of about 6.241509×10 18 electrons. That is a lot! Electrons have a charge of −1e and protons have a charge of +1e. Note: the elementary charge e .One coulomb is equal to the charge on 6.241 x 10 18 protons. The charge on 1 proton is 1.6 x 10 -19 C. Conversely, the charge of an electron is -1.6 x 10 -19 C. A coulomb is an .One coulomb consists of 6.24 × 10 18 natural units of electric charge, such as individual electrons or protons. From the definition of the ampere, the electron itself has a negative charge of 1.602176634 × 10 −19 coulomb.
Nob 3, 2020 — Electrons are tiny and have a very small charge. In physics, a very large number of electrons is defined as 1 unit of charge called a coulomb. One coulomb is the equivalent of 62 × 10 18 electrons. The .One electron possesses a charge of 1.6 × 10 − 19 C i.e., 1.6 × 10 − 19 C of charge is contained in 1 electron. ∴ 1 C of charge is contained in 1 1.6 × 10 − 19 electrons = 6.25 × 10 18 = 6 × 10 18 Therefore, 6 × 10 18 electrons constitute one coulomb of charge.
Ene 16, 2023 — The SI unit of charge is the coulomb, abbreviated C. One coulomb of charge is a lot of charge, so much that, two particles, each having a charge of +1 C and separated by a distance of 1 meter exert a force of \(9\times 10^9 N\), that is 9 billion newtons on each other. . neutral atom consists of a nucleus made up of neutrons and .

Ago 12, 2024 — Note: Coulomb is the standard unit for the electric charge. $1$ coulomb is the amount of charge present in an electric current of one ampere per second. The elementary charge $'e'$ is the charge carried by a single proton. It is a fundamental physical constant. So, charge of one electron constituting one coulomb of charge is .Ene 17, 2024 — The number of electrons constituting one coulomb of charge can be calculated using the elementary charge, denoted as “e,” which is approximately equal to 1.602 x 10^-19 coulombs. To find the number of electrons in one coulomb, we divide one coulomb by the elementary charge: Number of electrons = 1 coulomb / .
Recall that the charge on 1 mol of electrons is 1 faraday (1 F), which is equal to 96,486 C. We can therefore calculate the number of moles of electrons transferred when a known current is passed through a cell for a given period of time. . Use the definition of the faraday to calculate the number of coulombs required. Then convert coulombs .
Hul 23, 2018 — Can anyone tell the difference between = number-of-electrons- Coulomb and the formula definition {for example , since Speed = Distance/Time , so, 1 km/sec = 1 km covered in 1 second. (this type) }? . You can have 1/3 electron charge on subatomic particles. Share. Cite. Improve this answer. Follow answered Jul 23, 2018 at 14:19. A.J. .One coulomb charge is equivalent to the charge contained in: (a) 2.6 × 10 19 electrons (b) 6.2 × 10 19 electrons (c) 2.65 × 10 18 electrons (d) 6.25 × 10 18 electrons. View Solution. Q2. . The charge on a body is +1 C. Find the number of electrons in excess or deficit on the body.
a. A current of one ampere is a flow of charge at the rate of 1 coulomb per second. b. When a charge of 8 coulombs flows past any point along a circuit in 2 seconds, the current is 4 A. c. If 5 coulombs of charge flow past point A (diagram at right) in 10 seconds, then the current is 0.5 A. d. If the current at point D is 2.0 A, then 20 .A coulomb is the unit of charge, symbolized by C. One coulomb is the total charge carried by 6.25 X 10 18 electrons. A formula to calculate the coulombs of charge for a certain number of electrons is shown below: [latex]Q=\frac{Number of electrons}{6.25 X 10^{18}C}[/latex]
number of electrons in one coulomb of chargeHul 10, 2024 — Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal to 1.602176634 × 10−19 coulomb. In addition to the electron, all freely existing charged subatomic particles thus far discovered have an electric charge equal to this value
Charge of one electron = 1.602 × 10 − 19 coulomb Q. If the charge on an electron is 1.6 × 10 − 19 coulombs, how many electrons should pass through a conductor in 1 second to constitute 1 ampere current?All electrons and protons exhibit the same magnitude of charge, roughly 1.602E−19 coulombs. Thus, one coulomb is equivalent to the charge exhibited by approximately 1/1.602E−19, or 6.242E18 electrons. Further, opposite charges attract while like charges repel, similar to the poles of a magnet. It is possible to move charge from one point to .Peb 26, 2016 — Here's a chemistry perspective. Faraday's number gives the charge held by 1 mole of electrons: 96,485 C. Therefore, 1 Coulomb is 1/96485 moles of electrons, or around $10^{-5}$ moles. Based on this, you can deduce that any corroded object of a reasonable size formed by passing at least 1 Coulomb of charge.
How many electrons are there in 1 coulomb? A collection of 6.2415 times 10 raised to the 18th power would have a charge of approximately -1 Coulomb. Tom (published on 10/22/2007) Follow-Up #1: How many electrons in one Coulomb. Q: . The Millikan oil drop experiment which measures the charge on a single electron provides that answer. (There are more modern .If total charge = 1 C. . How many electrons are there in one coulomb of electricity? View Solution. Q5. How many moles of electrons weighs one kilogram? [Mass of electron = 9.108 × 10 − 31 k g, Avogadro's number = 6.023 .Ago 23, 2024 — Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electron charge, equal to $1.602176634 \times {10^{ - 19}}$ coulomb. We need to calculate the number of electrons constituting one coulomb of .The coulomb, also written as its abbreviation 'C', is the SI unit for electric charge. One coulomb is equal to the amount of charge from a current of one ampere flowing for one second.. One coulomb is equal to the charge on 6.241 x 10 18 protons. The charge on 1 proton is 1.6 x 10-19 C. Conversely, the charge of an electron is -1.6 x 10-19 C. . A .
333 Ängelnummer betydelse i olika livssituationer. I helig numerologi representerar siffran 333 lycka. Du måste komma ihåg exakt var du märkte detta meddelande från skyddsängeln. Till exempel, om du har lagt märke till nummer 333 på ett fordons registreringsskylt, har du lycka till på din resa. Om husnumret du ser är 333 .
number of electrons in one coulomb of charge|How many electrons are there in 1 coulomb?